ex-vivo gene therapy
data set in the world.
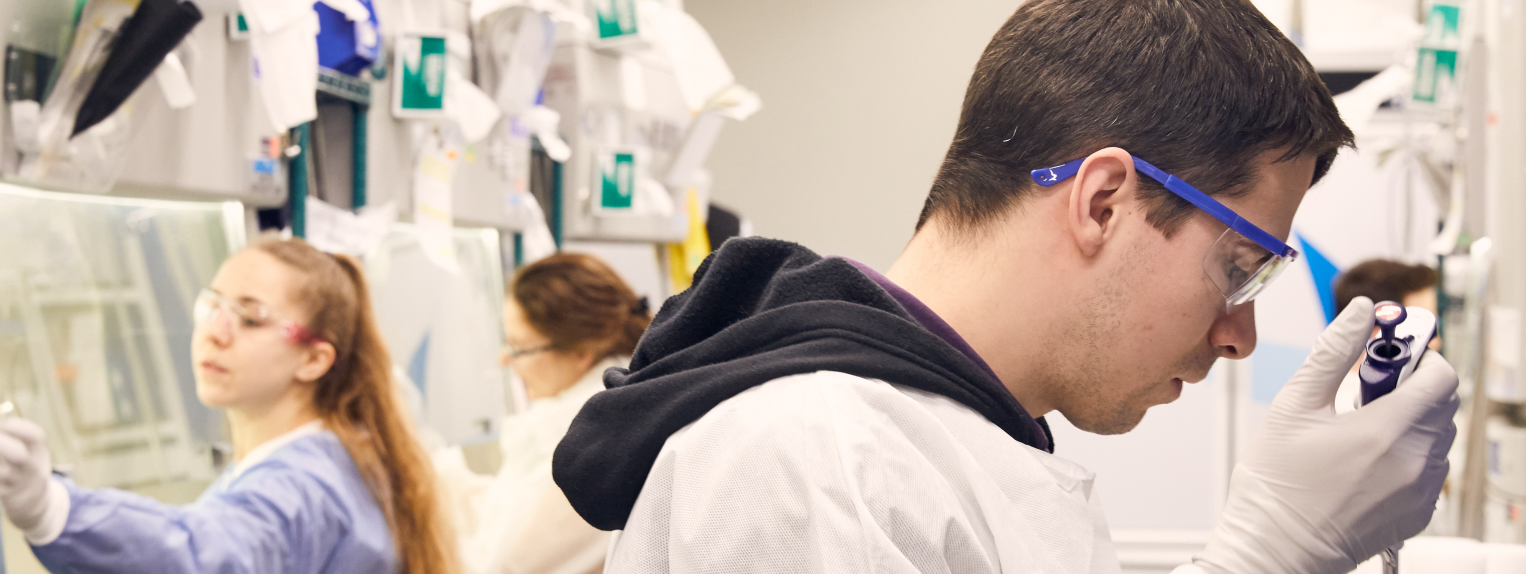
our science
With more than a decade of expertise and over 500 patient-years of experience, we are driving the field forward and setting the standard for gene therapy. Our lentiviral vector (LVV) technology is applicable to a broad range of serious diseases and has the potential to provide transformative benefits.
We are focused on developing and delivering new therapies for severe genetic diseases that currently have limited or no treatment options. Our goal is to provide hope for a better life – and more bluebird days – for patients and their families.
what sets us apart?
- Largest and deepest ex-vivo gene therapy data set in the world
- >500 patients-years of experience across all LVV gene therapy clinical studies
- 170+ patients studied across 8 clinical trials with up to 7 years of follow-up
what is gene therapy?
Gene therapy uses genetic material with the goal of changing the course of a disease by adding, deleting, or correcting a genetic mutation. Gene therapy is being studied for a number of diseases, including inherited diseases and cancers. Today, building on more than a century of research into the building blocks of DNA, there are an unprecedented number of clinical trials underway with gene therapies and some have already been approved by the US Food and Drug Administration (FDA).
By addressing diseases at their root cause, gene therapy has the potential to transform the treatment of many diseases.
National Institutes of Health. Genetics Home Reference. Help me understand genetics.
Wirth T, Parker N, Ylä-Hertuala. History of gene therapy. Gene. 2013;525(2):162-169.
Food and Drug Administration. Approved Cellular and Gene Therapy Products.
Dunbar CE, High KA, Joung JK, Kohn DB, Ozawa K, Sadelain M. Gene therapy comes of age. Science. 2018;359(6372):eaan4672-eaan4672.
Negre O, Eggimann A-V, Beuzard Y, et al. Gene therapy of the β-hemoglobinopathies by lentiviral transfer of the βA(T87Q)-globin gene. Hum Gene Ther. 2016;27:148-165.
Morgan RA, Gray D, Lomova A, Kohn DB. Hematopoietic Stem Cell Gene Therapy: Progress and Lessons Learned. Cell Stem Cell. 2017;21(5):574-590.
Goswami R, Subramanian G, Silayeva L, et al. Gene therapy leaves a vicious cycle. Front Oncol. 2019;9:297.
Bone Marrow & Cancer Foundation. Your Transplant Journey. Available at: https://bonemarrow.org/images/documents/Your-Transplant-Journey---Bone-Marrow--Cancer-Foundation.pdf. Accessed April 2022.</https:>